Enterprise Learning Management Software Empowering Agility and Compliance
Meet PeopleFluent Learning, a powerful and configurable LMS purposefully built for large enterprises that are focused on compliance and competencies—now with a modular front-end experience for your end-users.
Take your L&D strategy to the next level with AI-powered skill development and workforce agility. Close skills gaps, enable employee development, and make your workforce more agile.
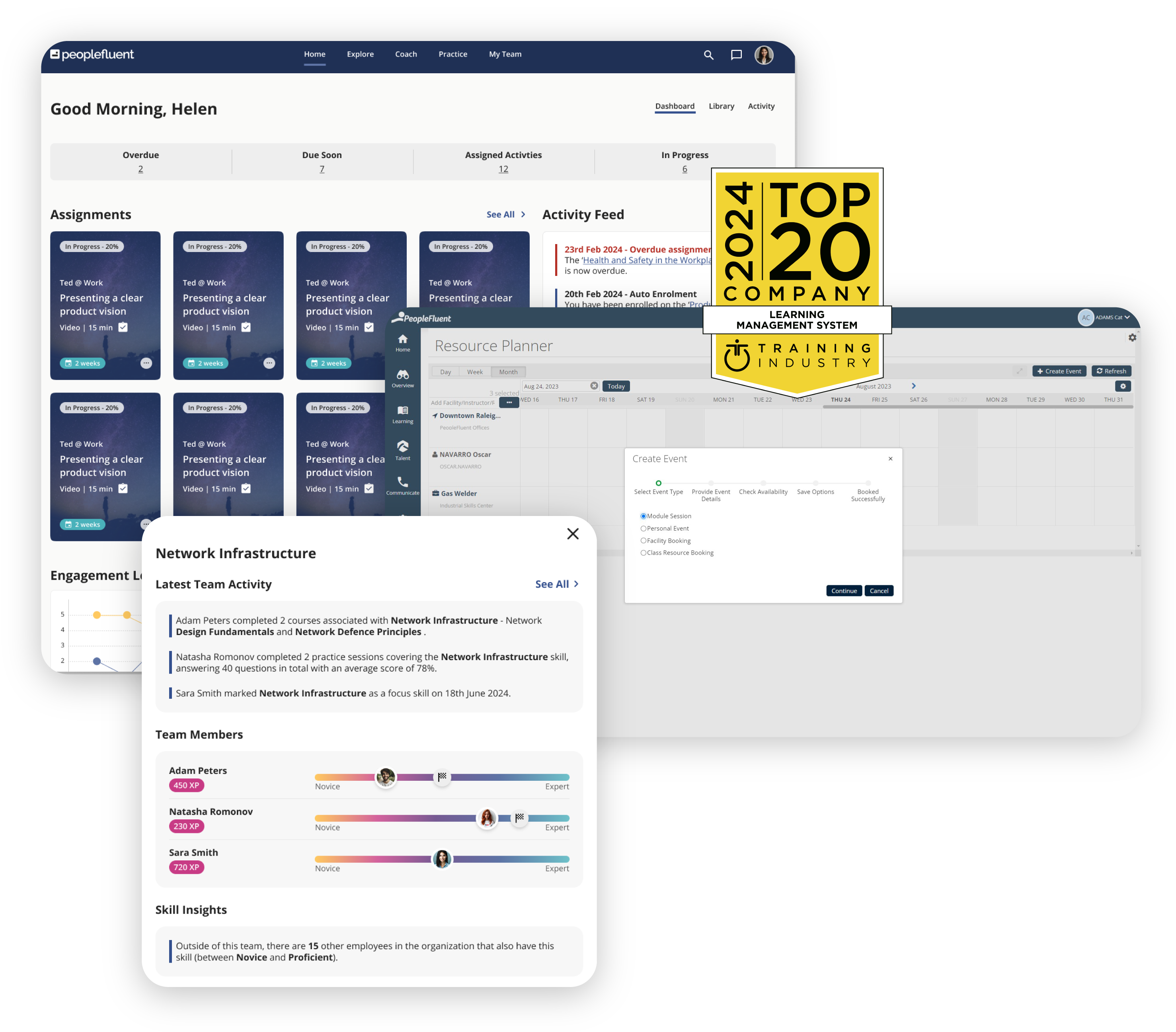
Develop Employees. Upskill Talent. Maintain Compliance.
Stay ahead of the curve with an industry-leading LMS. PeopleFluent Learning makes it easy for your organization to:
- Successfully deliver learning and development programs
- Drive measurable employee development outcomes with personalized learning paths
- Ensure consistent workforce growth and compliance
- Build a highly skilled, compliant workforce
- Encourage and foster a culture of continuous learning
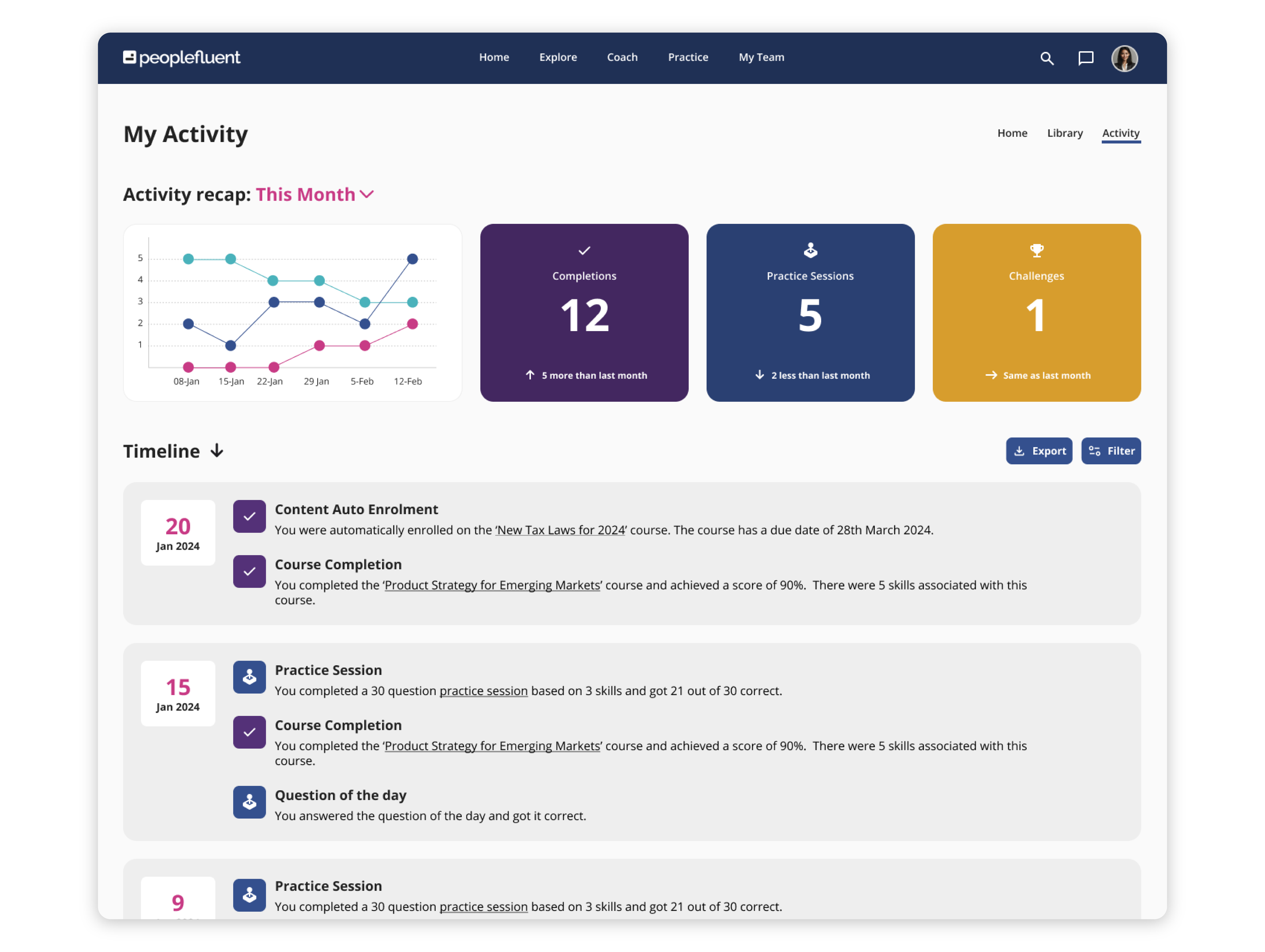
Empowering for Learners. Effortless for Admins. Revolutionary for Business.
PeopleFluent Learning specializes in compliance for global, highly regulated industries and is tailored to meet your enterprise-scale needs. Our on-premise and cloud-based solutions centralize your L&D efforts, provide real-time data tracking, and offer a highly configurable learning platform that’s aligned with your business goals. With PeopleFluent, you can:
- Keep all your compliance training and learning materials in one convenient place
- Streamline your content delivery
- Maintain brand consistency across learning portals
- Easily integrate third-party content and off-the-shelf courses
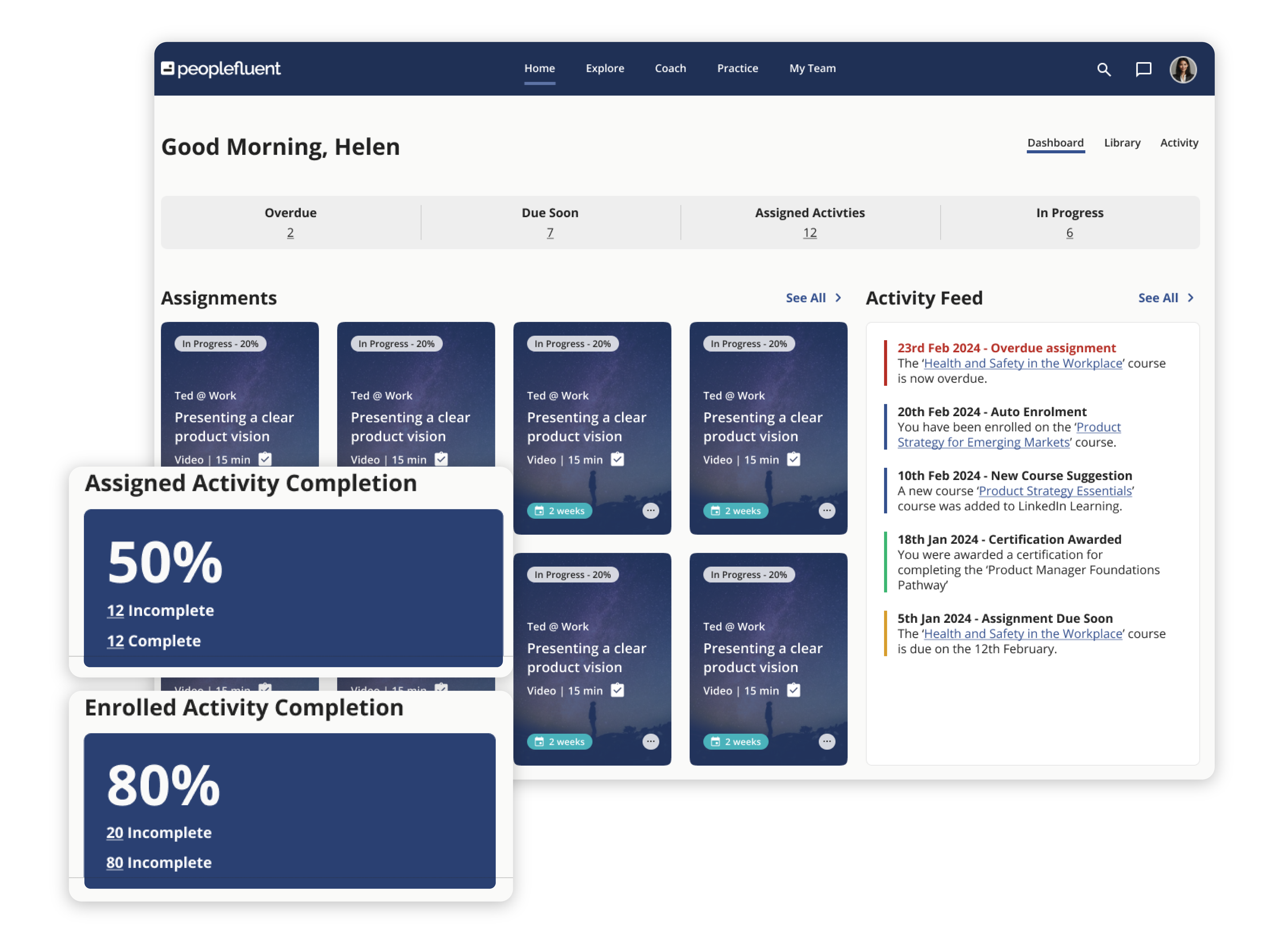
Discover what PeopleFluent Learning has to offer:
Learning and Development Essentials
PeopleFluent Learning includes the features and tools every L&D program needs to succeed. With PeopleFluent as your LMS provider, you can easily:
- Create, manage, and deliver courses and eLearning material to your learners
- Store key information on each learner’s journey and performance
- Track training courses and certifications with robust analytics
- Tie compliance training and employee development to measurable business outcomes with skills-based and competency-based training
- Enable skills gap identification so your workers know where they stand
- Highlight targeted training courses that help employees close knowledge gaps
- Build assessments that include comprehensive tracking to understand the effectiveness of your programs over time
- Support employee development with personalized learning paths and visually engaging progress monitoring
- Employ advanced triggers and email options for personalized and localized course-related communications
PeopleFluent Learning is trusted by top global brands
Join organizations around the world that trust PeopleFluent with their learning and development needs.


with enrolled learners

for the L&D department

supported in 12 countries
completion rate
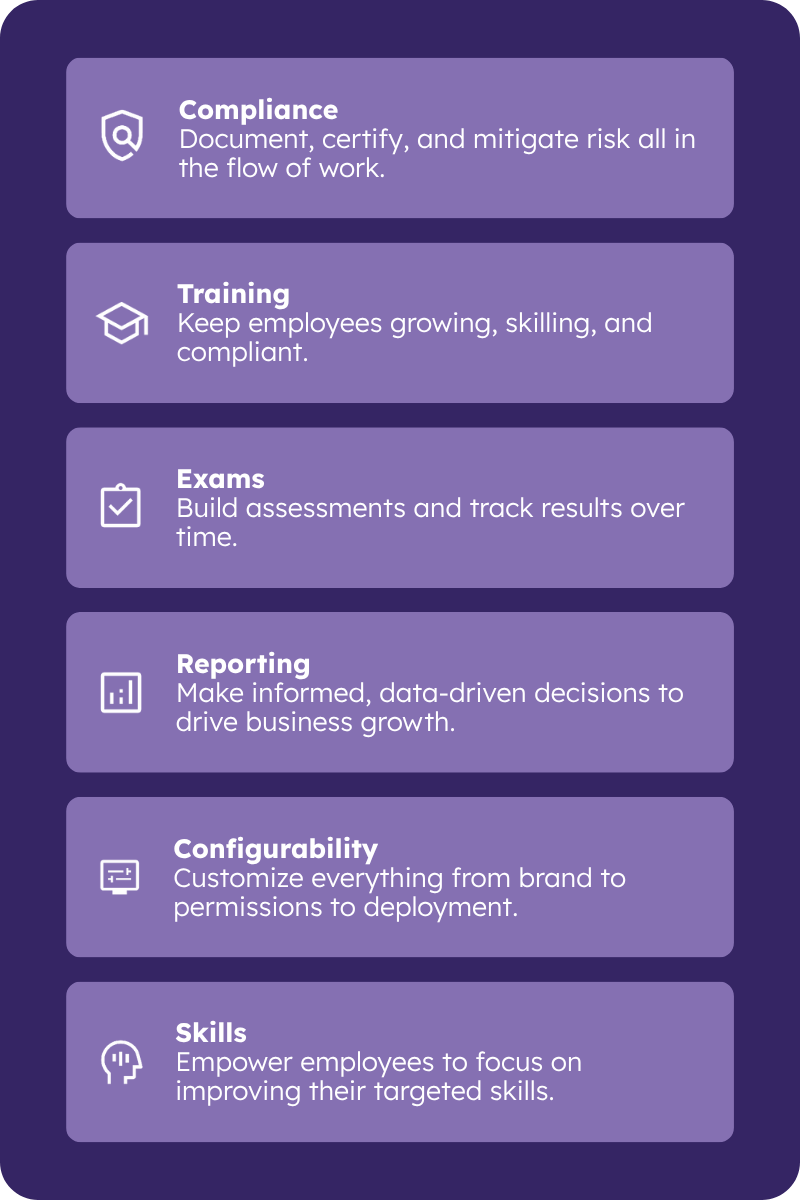
Comprehensive Compliance Management
When your bottom line depends on keeping staff both actively working and up to date on compliance issues, the right learning management technology can be the difference between keeping your business running or running into regulatory hot water. If your organization operates in highly regulated industries, then you need compliance training support that goes beyond standard LMS functionalities.
Comply with complex regulatory processes and reduce the risk of error with PeopleFluent Learning’s automated compliance management features. Let the software do the heavy lifting so you don’t waste unnecessary time preparing for audits---mitigating risks and ensure your organization maintains regulatory compliance. Take advantage of robust management tools, including:
- The ability to create and define custom user roles
- Granular controls for tailoring administrative, manager, and user privileges
- Specific course communications with nearly 30 notification triggers and over 200 course-related dynamic variables
- Support for more than 40 languages, including right-to-left languages Arabic and Hebrew
- Hierarchical and matrix managerial structures for reviewing and updating training plans
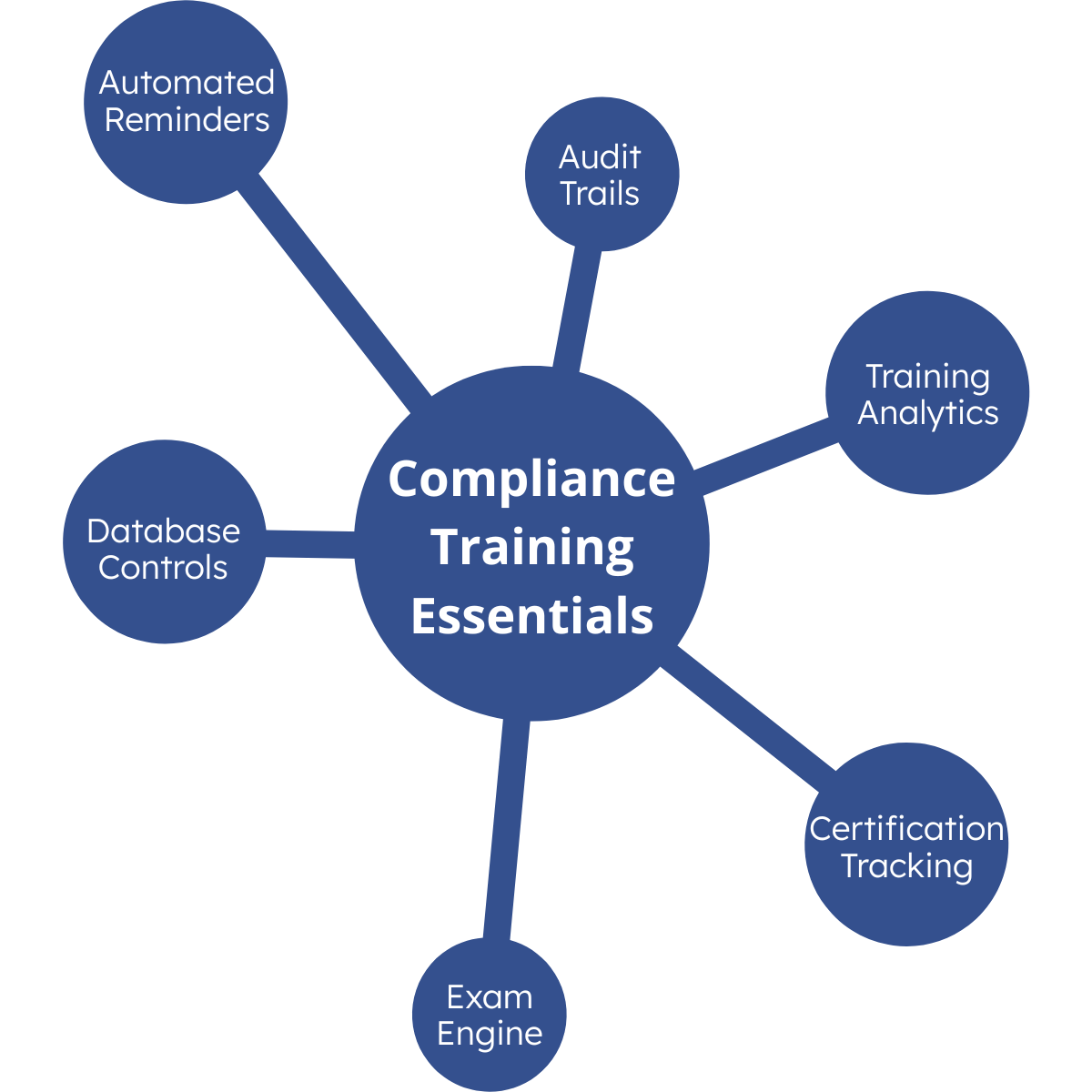
Why PeopleFluent?
Discover L&Q Group’s L&D Transformation With PeopleFluent Learning
Case Study
L&Q Group, one of the UK’s largest housing associations, streamlined its compliance training content delivery with PeopleFluent Learning’s automation features.

Built for Regulated Industries

Aviation
Streamline pilot certifications, and crew and maintenance training.

Energy
Track safety protocols and environmental compliance.
Financial Services
Ensure up-to-date regulatory knowledge across teams.

Healthcare
Manage continuing education and patient safety training.

Life Sciences
Maintain GxP compliance and research ethics standards.

Manufacturing
Coordinate quality control and safety procedure training.

Pharmaceuticals
Oversee clinical trial education and drug safety protocols.

Transportation
Monitor licensing requirements and safety regulations.
Case Study
Humentum’s Global Learning Impact With PeopleFluent Learning



Powerful Reporting and Analytics
Leverage real-time insights to optimize your compliance programs and maximize your training ROI. PeopleFluent Learning’s analytics suite shows you engagement gaps and helps you measure your program effectiveness. Make data-informed decisions and take the guesswork out of your L&D programs with features like:
- Real-time analytics
- More than 150 standard reports
- Custom report tool
- Automatic report scheduling, including sending via Secure File Transfer Protocol (SFTP)
- Detailed tracking for course completion, certifications, competencies, and assessment grading
Case Study
How a Large UK Financial Institution Improved Compliance Training Metrics


On-Premise or Cloud-Based for Seamless Integration and Accessibility
PeopleFluent supports on-premise and cloud-based LMS implementation, ensuring your organization’s unique L&D needs are met. Stay agile and responsive to changing learning demands, maintain robust security and compliance standards, and create a seamless experience for your teams with LMS hosting options that work for your business. PeopleFluent Learning makes it easy to:
- Connect with your existing HR systems
- Support your growing needs with scalable infrastructure
- Stay up to date with automatic updates and regular maintenance
- Protect your sensitive training data with enhanced security features
- Access on-the-go learning and training materials via a mobile-ready platform
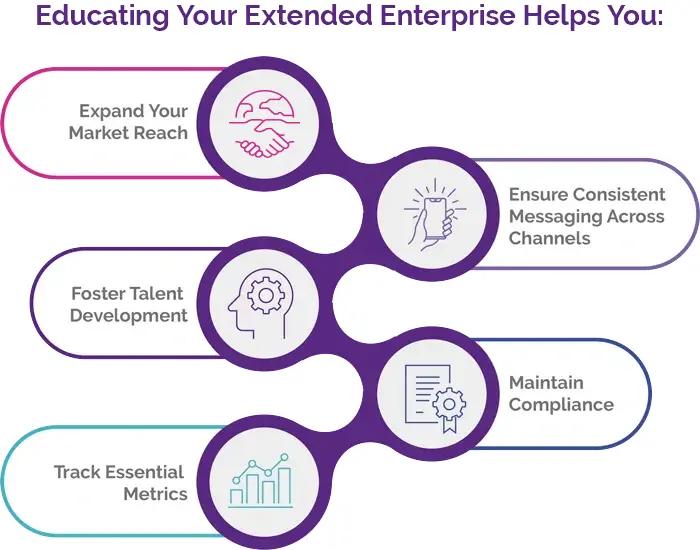
Solutions for Extended Enterprise
Take your training programs beyond your internal workforce. Educate vendors, partners, and other external stakeholders and expand your training ecosystem with PeopleFluent Learning’s configurable training experiences. Take advantage of these opportunities to extend your sales enablement efforts:
- Partner Training: Equip suppliers, resellers, franchise owners, and other business partners with the knowledge they need to represent your brand accurately and effectively.
- Customer Education: Offer product training and certification programs to enhance customer satisfaction and loyalty.
- Vendor Management: Ensure supply chain vendors meet your quality standards through targeted training initiatives.
- Franchise Support: Maintain consistency across your franchise network with standardized onboarding and ongoing education.
- Revenue Generation: Transform your learning programs into profitable ventures by selling courses to external customers.
Case Study
PeopleFluent helped Norton Healthcare improve its compliance reporting and enhance user experiences.



PeopleFluent: Your Partner in Continuous Learning

Skill and Competency Management
Identify learners’ skills gaps, and foster a culture of continuous learning with targeted training courses that help employees close those gaps.

Exam Engine
Administer and evaluate training on multiple levels, such as all four levels in the Kirkpatrick Model. Decide who can access exams, how they’re graded, when they can be reviewed, and who can review them.

Configurability
Customize the system interface and settings, notification rules and triggers, and in-depth permissioning and user roles.

Skill Reinforcement
Periodically check knowledge retention through both human- and AI-generated practice quizzes, with real-time results, as well as conversational-style AI coaching.

Learner Motivation
Enhance the learner experience through gamification, awards, and challenges. Highlight gaps in learner skills and recommend related training to make improvements.

Recommended Learning
Find personalized AI-generated recommendations based on learners’ skills and proficiency levels, as well as administrator-curated suggested courses.
PeopleFluent Learning FAQ
Get the answers to the most frequently asked questions about PeopleFluent’s LMS.
How quickly can PeopleFluent Learning be implemented?
What level of support can we expect after implementation?
Can PeopleFluent Learning handle complex organizational structures?
How does PeopleFluent Learning ensure we stay compliant across different regions?
How does PeopleFluent Learning drive user engagement?
How does PeopleFluent compare to other LMS platforms?
Enterprise Learning Management Resources
It’s Time to Transform Your Learning Strategy
Join enterprise organizations around the world that have enhanced their workforce agility, training programs, and talent development strategies with PeopleFluent Learning. Request your personalized demo today: